The Science Behind Fast Food Fries

Why the **** does McDonalds have 19 ingredients in their fries? That was my initial thought when I first learned about it in my very productive, enjoyable Kinesiology class. The idea that French fries could contain more than 2 ingredients appalled me, and I researched it further to find any justification for my love for McDonald’s fries. What I found out was honestly quite interesting.

Initially, I only looked at McDonald’s fries, but as I ventured down the rabbit hole, Shake Shack fries also caught my attention. Honestly, a lot of food science goes into making the perfect fry, and I’ll explain to you guys some of it today. But first, here’s a list of the 19 ingredients in McDonalds fries:
1. Potatoes
2. Canola Oil
3. Soybean Oil
4. Hydrogenated soybean Oil
5. Natural Beef Flavour
6. Hydrolyzed wheat
7. Hydrolyzed milk
8. Citric acid
9. Dimethylpolysiloxane
10. Dextrose
11. Sodium acid pyrophosphate
12. Salt
13. Tertiary butylhydroquinone
There aren’t actually 19, as some of them are repeated, but 13 does seem to be an extravagant amount. However, it appears more reasonable when you consider that ingredients 2 – 12 are utilized in the oil to fry the fries. McDonald's fries are double fried, once in their mass production and again at the restaurant. Still, there are compounds here that I’d bet university-level chemistry students don’t know about.
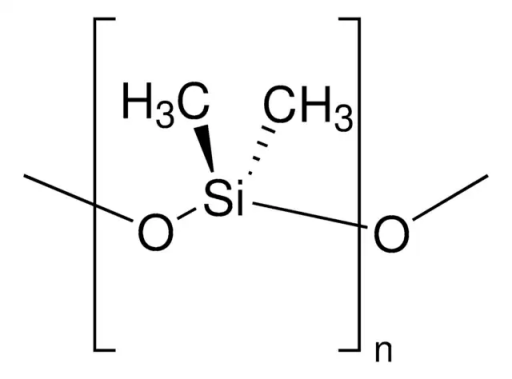
Dimethylpolysiloxane is a silicone polymer with several uses, from cosmetics to food. Here, it’s added due to its good defoaming effectiveness and ability to prevent oil splatters during frying. Contrasting its frightening name, it’s a food additive approved by the FDA, EFSA, and several other food authorities.

Fundamentally and chemically, oil is made up of carbon and hydrogen atoms. Oxidation occurs when a hydrogen atom is knocked off and replaced with an oxygen atom. This can happen through heat (frying), which changes the oil's flavour, colour, and nutritional value. Antioxidants prolong the induction period of automatic oxidation for oils, which is what citric acid is. Therefore, it extends the lifetime of frying oils, allowing restaurants to prevent any accidental spoilage or rancidity. 2006
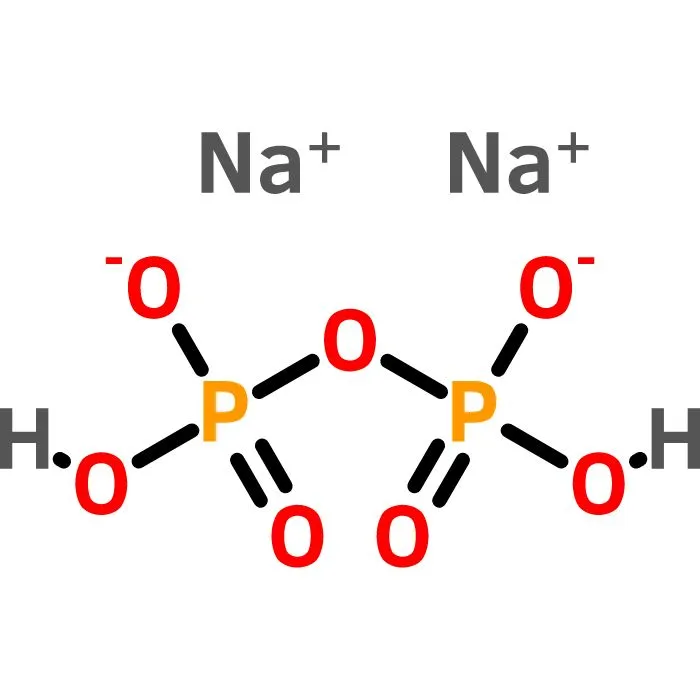
While the past 2 compounds have been used as additives to the oil, sodium acid pyrophosphate is utilized to maintain the colour of the fries. No one wants black fries; that beautiful golden colour is just much more appetizing. The discoloration happens due to a chemical reaction between iron and chlorogenic acid, natural components of a potato. Sodium acid pyrophosphate binds to iron and prevents this reaction, maintaining the desired colour.

If frozen fries require so many additional ingredients, why don’t fast food chains make them fresh? Simply put, it’s more convenient. Shake Shack tried to make fresh fries, where chefs would peel, cut, and fry the potatoes in the restaurant. This took hours, and customers wanted the original crinkle fries back. Fresh fries were off the table and Shake Shack’s emphasis on freshness looked down upon frozen fries. So, as Mark Rosati, the Culinary Director of Shake Shack, tried to find the perfect recipe, he visited British Chef Heston Blumenthal, known for his delicious fries. Blumenthal actually used frozen potatoes, as they lock all the moisture in the fry, and when you fry them in that state, the moisture explodes and creates a delicate and soft interior. What did Rosati realize? That frozen isn’t a bad word among fries.
Food science is a lot more complicated than it appears, with the simple French fry requiring meticulous amounts of planning and execution. If it was to come to light that McDonalds put heinous amounts of drugs in their fries, I wouldn’t be surprised, they’re that good. And I’d still eat them.
Thanks for reading, everyone, and Happy Chinese New Year!